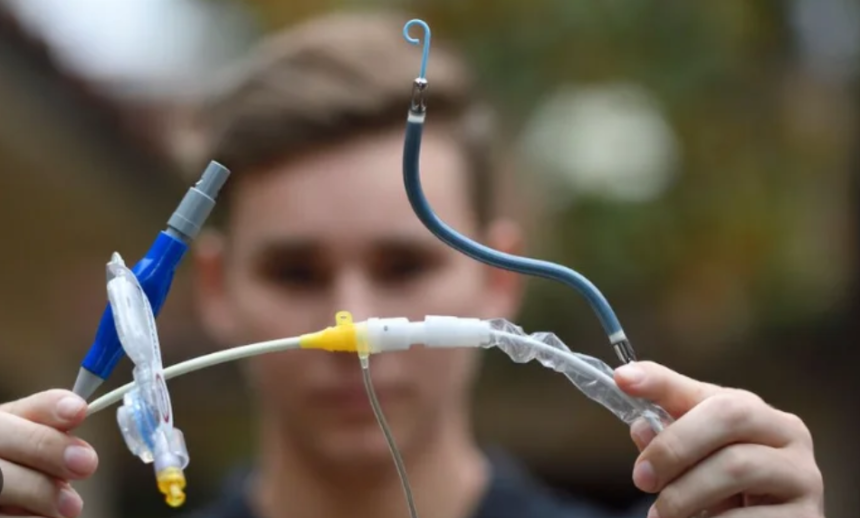
A fresh alert has been released regarding an abiomed heart pumps whose usage may puncture the heart.
49 fatalities and more than 100 injuries have already been connected to the device.
The manufacturer of these left-sided Impella heart pumps is Abiomed, a division of Johnson & Johnson MedTech. The U.S. Food and Drug Administration website has updated cautions about the devices that Abiomed posted.
“This recall falls under Class I, the most dangerous category, according to the FDA. The statement stated that “using these devices may result in serious injuries or death,” but it also added that “this recall is a correction, not a product removal.”
Also read-A New Federal Regulation: Written Consent Is Required For Pelvic And Prostate Exams In Hospitals
Abiomed Heart Pumps
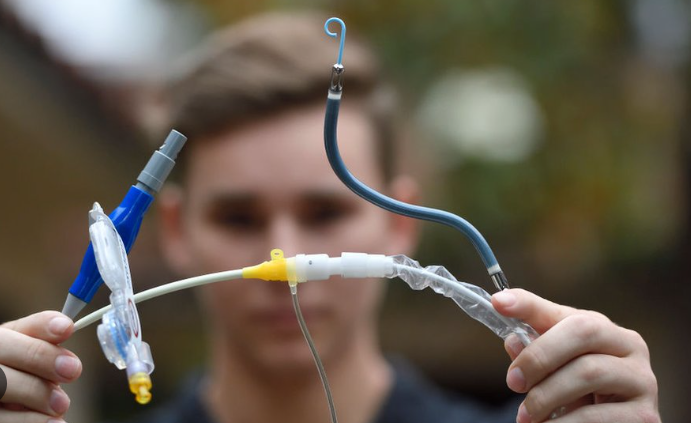
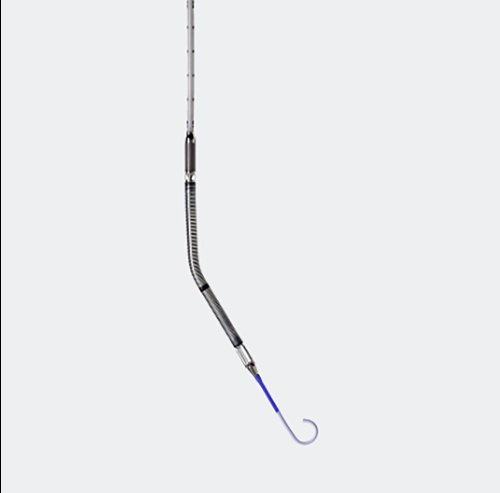
The advisory advises implanters of Impella devices to follow updated usage guidelines, which include the advice to “carefully position the pump catheter during operative procedures.” The Impella pumps are similar to a long straw that has been put inside the heart. They assist in preserving appropriate blood flow from the heart to the body during high-risk cardiac procedures (such as some types of heart attacks).
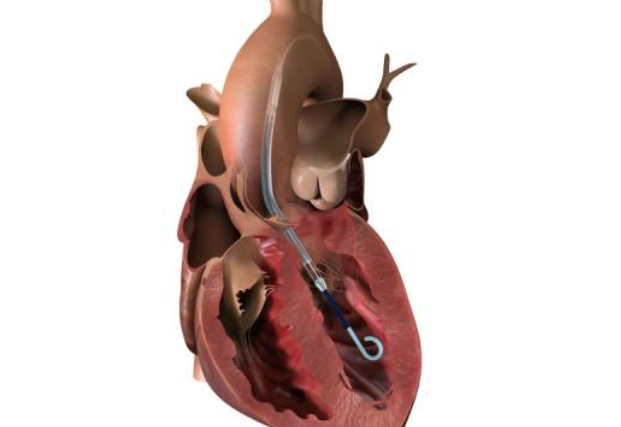
The pump is inserted into the left ventricle, the heart’s primary pumping chamber, via the main cardiac arteries. “Abiomed is recalling its Impella Left Sided Blood Pumps because the pump catheter may perforate [cut] the wall of the left ventricle in the heart,” the business stated in a statement. “During operations, the Impella device could cut through the wall of the left ventricle.”
Abiomed Heart Pumps
“The use of the affected Impella pumps may cause serious adverse health consequences, including left ventricle perforation or free wall rupture, hypertension, lack of blood flow and death,” the advisory stated.
49 people have passed away and 129 patients have reported significant injuries connected to the devices thus far.

Abiomed Heart Pumps
In October 2021, a technical bulletin to physicians revealed the problem for the first time, although the FDA was not notified at that time. CNN was informed by an FDA spokesman that doing so would have been against agency policy.
In September 2023, the FDA inspected Abiomed’s facilities as a follow-up and promptly sent the company a warning notice.
Abiomed Heart Pumps
The most recent Abiomed advisory states that patients having procedures with Impella Left Sided Blood Pumps should be aware of the updated usage instructions, particularly those who have heart disease, are elderly, or are female.
However, a consumer advocacy group called Public Citizen released a statement demanding an outright prohibition on gadgets.

Abiomed Heart Pumps
“The FDA has allowed them to remain in use,” the group added, despite dozens of serious injuries and fatalities. “Moreover, there are serious and ongoing concerns about whether there are clinically meaningful survival benefits that outweigh the risks of these left ventricular assist devices.”
The head of the Public Citizen’s Health Research Group is Dr. Robert Steinbrook.
“Given the ongoing safety concerns about Impella left ventricular assist devices and this new recall, it is woefully inadequate to revise an instruction manual and to tell cardiologists to be more careful,” Steinbrook stated in a statement. “The use of these left ventricular assist devices should be stopped.”
Going forward, Steinbrook stated, “These devices should only be used in patients enrolled in randomized, controlled trials that compare the devices to medical [drug] management.” “Better treatments are urgently needed,” he continued.
Also read: The Law On Free Access To Morning After Pill For Those Over 15 Is Vetoed By The President Of Poland
images source: Google
Disclaimer: The opinions and suggestions expressed in this article are solely those of the individual analysts. These are not the opinions of HNN. For more, please consult with your doctor