The Earth’s atmosphere, a complex mix of gases that envelops our planet, plays a vital role in supporting life and regulating climate. One of the intriguing aspects of the atmosphere is the absence of mass-based gas separation, meaning that gases are not neatly layered according to their molecular weight. In this article, we will explore the reasons behind this absence and the factors that maintain the atmosphere’s unique composition.
Earth Atmosphere
As the image below illustrates, the many gases that make up the earth’s atmosphere do, in fact, partially divide into strata based on molecular mass. The atmosphere’s composition is depicted in this image in relation to altitude above Earth. Each curve in this picture has a label that identifies the gas it represents as well as the estimated mass of one molecule or one atom of that gas, depending on the situation, in unified atomic mass units.For example, atomic oxygen (O) is represented by the blue curve, and the mass of one oxygen atom is approximately 16 u. The most substantial common gases, argon, molecular oxygen, and nitrogen, are shown in this image to have their maximum concentrations in the lower to intermediate atmosphere. The intermediate to upper atmosphere has the highest amounts of the intermediate-mass common gases, atomic nitrogen and oxygen. The highest concentrations of the lightest common gases, hydrogen and helium, are found in the upper atmosphere.
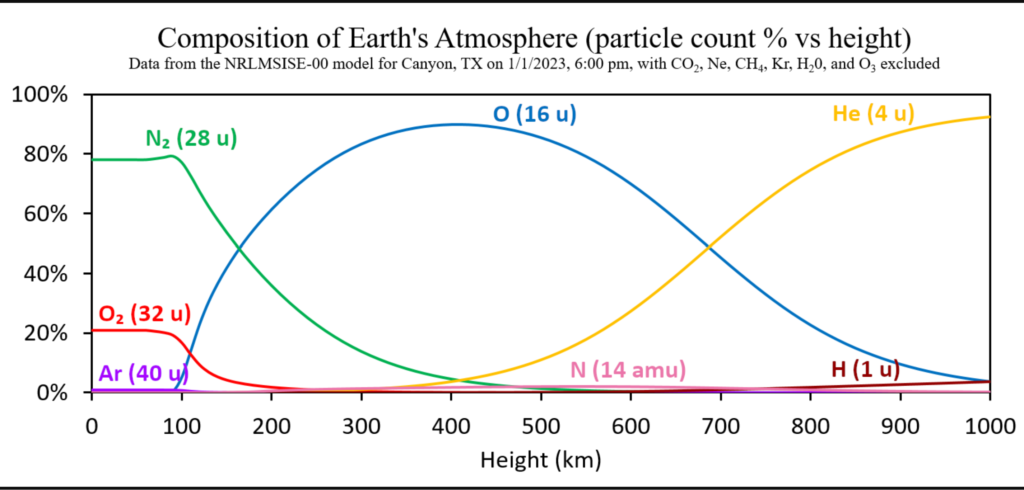
The composition of earth’s atmosphere as a function of height, showing the partial separation of the gases according to molecular mass. This data set was obtained from the NRLMSISE-00 model for Canyon, TX on January 1, 2023 at 6:00 pm. Click on the image to enlarge it. Public Domain Image, source: Christopher S. Baird.
The troposphere, the lowest layer of the earth’s atmosphere, rises to a height of roughly 15 km from the surface. The majority of clouds, weather patterns, and convection systems on Earth are found in the troposphere. In addition, the majority of nitrogen (N2), oxygen (O2), and argon atoms (Ar) are found in the troposphere. But as I’ll explain later, they are constantly compelled to mix violently with one another, resulting in essentially zero molecular mass separation of these gases in the troposphere. As a result, as the following chart illustrates, the relative amounts of these gases remain constant throughout the troposphere.
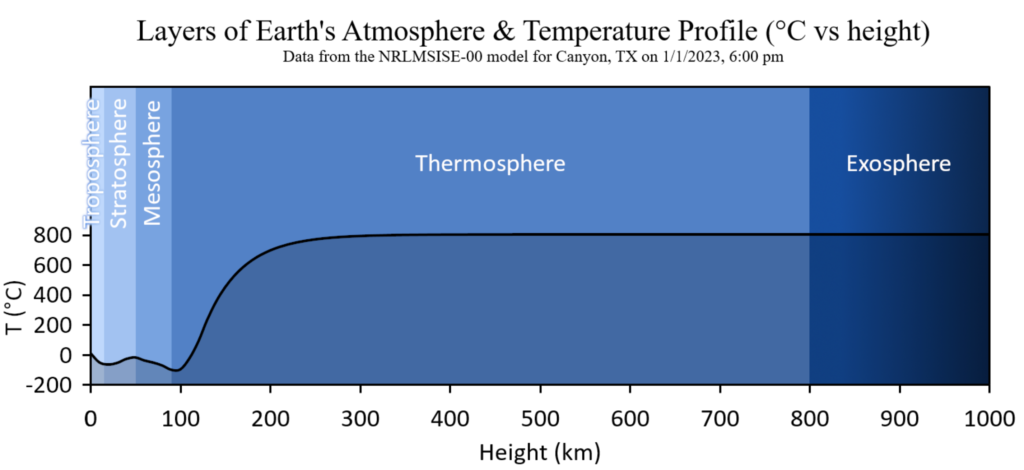
The main layers of earth’s atmopshere and the temperature profile. This data set was obtained from the NRLMSISE-00 model for Canyon, TX on January 1, 2023 at 6:00 pm. Click on the image to enlarge it. Public Domain Image, source: Christopher S. Baird.
Stratosphere
The stratosphere, which rises to a height of roughly 15 to 50 km, lies above the troposphere, and the mesosphere, which rises to a height of roughly 50 to 90 km, lies above that. The figure immediately above illustrates the many levels of the atmosphere. The highest-flying aircraft and weather balloons fly in the stratosphere. The majority of meteors ignite and break apart in the mesosphere as they descend. You can see that the amounts of molecular nitrogen, molecular oxygen, and argon in the troposphere, stratosphere, and mesosphere are roughly equal by comparing the two figures above.Because of this, the gases in all three of these layers are uniformly mixed and do not segregate based on molecular mass. This is a result of the air in these three levels being vigorously agitated. Because of this, the homosphere refers to the lowest three layers of the atmosphere as well as the bottom edge of the thermosphere. The ozone layer, which is not evenly distributed throughout the homosphere, is the only exception to this rule. Nevertheless, chemical reactions—rather than a division of gases based on molecular mass—are to blame for this.
Thermosphere
Within the thermosphere, which spans from 90 km to 800 km, things get really intriguing. In the thermosphere, aggressive mixing effects such as thermal convection are negligible. As the first figure above illustrates, the gases in the thermosphere can thus partially split based on molecular mass. The gases are not entirely separated based on molecular mass, though. The reason for this is molecular diffusion.Atomic oxygen (O) and molecular nitrogen (N2) make up roughly equal proportions of the thermosphere’s lowermost layer. Atomic oxygen (O and O+) makes up almost all of the thermosphere’s midsection. A portion of hydrogen (H) and helium (He+) are present in the upper thermosphere, along with roughly equal amounts of atomic oxygen (O and O+) and helium (He and He+). Observe that the ionised form of the atoms becomes just as frequent as the neutral form at the middle of the thermosphere. The majority of atoms are ionised above the middle thermosphere.
Exosphere
The exosphere, which stretches from roughly 800 km to roughly halfway to the moon, or roughly 190,000 km, is the outermost region of the atmosphere. Because there is essentially no forceful mixing in the exosphere, the gases partially separate apart based on mass.Ionised helium (He+) makes up the majority of the lowest region of the exosphere. A little above this lowest point, which is hidden in the first diagram above, are roughly equal amounts of ionised hydrogen (H+) and helium (He+). Nearly all of the exosphere is made up of ionised hydrogen (H+), which is not depicted in the first diagram above. Keep in mind that treating the thermosphere and exosphere as components of space is beneficial in many situations.
The NRLMSISE-00 model provided the information for the two figures above. The atmospheric conditions above Canyon, Texas on January 1, 2023 at 6:00 p.m. are represented by these data sets. While there are tiny fluctuations in the status of the atmosphere from hour to hour, day to day, and place to location, the data will always appear very similar to the figures above.It should be noted that the first picture above does not include ozone (O3), water vapour (H2O), krypton (Kr), methane (CH4), neon (Ne), or carbon dioxide (CO2). This is because these molecules and atoms are too infrequent to be visible in this figure at most heights. For example, although the concentration of ozone molecules in the ozone layer is rather high, they still only constitute 0.001% of the atmosphere in this layer—a quantity that is far too little to be shown in the first diagram above.
Three factors make the molecular mass separation of the atmosphere’s gases only partially complete: (1) the atmosphere’s lowest three layers are constantly and violently mixed; (2) chemical reactions alter the gases; and (3) molecular diffusion contributes to some mixing, even in the thermosphere and exosphere.
As a result of vigorous gas mixing, chemical reactions, and molecular diffusion, the various gases in the atmosphere do, in fact, partially separate apart according to molecular mass due to gravity. However, this separation is only partial. In the homosphere, which includes the troposphere, stratosphere, mesosphere, and lower edge of the thermosphere, aggressive mixing is the predominant process; in the heterosphere, which includes the exosphere and the majority of the thermosphere, molecular diffusion is the dominating mechanism.
FAQ
Q: Why don’t heavier gases sink to the lower layers and lighter gases rise to the upper layers in the atmosphere?
A: This phenomenon is due to a fundamental process called molecular diffusion. It ensures that gases are constantly mixed throughout the atmosphere, preventing mass-based separation. Even though heavier gas molecules move more slowly than lighter ones due to their greater mass, they still exhibit random motion, resulting in the constant mixing of gases.
Q: Can mass-based gas separation occur under certain conditions?
A: Yes, in confined environments with minimal mixing, gases can separate based on their molecular weight. For instance, in a closed container, heavier gases can settle at the bottom while lighter gases rise to the top. However, in the open atmosphere, molecular diffusion and turbulent mixing counteract gravitational separation
Q: Does mass-based separation have any practical applications on Earth?
A: Gravitational separation of gases based on their molecular weight is exploited in some industrial and scientific processes. For example, it is used in the production of liquefied air products and the separation of gases for specific applications.
Q: How does mass-based separation of gases occur on other celestial bodies?
A: Mass-based separation can occur on celestial bodies with different atmospheres. For example, the gas giant Jupiter exhibits distinct layers due to the separation of heavier and lighter gases. Venus, Earth’s neighboring planet, has a predominantly uniform atmosphere, albeit with an extreme greenhouse effect due to the abundance of carbon dioxide.
ALSO READ :What Happens When A Spaceship Flies At The Speed Of Light? Lets Find Out




































