Earth is special because of its ozone layer. The colourless gas known as ozone is composed of three oxygen atoms.Six to fifty kilometres up in the stratosphere is where most ozone is found. But the majority of ozone is found between 6 and 10 kilometres.For humans to prevent skin cancer, ozone is essential. Additionally, it’s critical for illness prevention and animal health.
How was the ozone layer formed?
Here are the necessary steps for ozone to form in the atmosphere:
OXYGEN PRESENCE: Oxygen in the atmosphere was the initial requirement for the formation of an ozone layer on Earth. This was made possible by the Great Oxygenation Event.
SINGLE OXYGEN ATOMS:Certain oxygen (O2) molecules absorb solar ultraviolet (UV) radiation high in the atmosphere. As a result, ozone separates into individual oxygen atoms.
OZONE FORMATION: These atoms combine with molecular oxygen (O2) to form ozone (O3) molecules. These ozone molecules are very effective at absorbing UV rays.
Also read: Clouds Are Just Water Vapour, So Why Do They Move?
How is ozone distributed in the stratosphere?
Over the world, ozone has different thicknesses. Additionally, it varies according to the seasons and atmospheric circulation.
In the stratosphere, ozone is concentrated between 15 and 35 kilometres up. You would think that the majority of stratospheric ozone would be found around the equator as UV radiation acts as an ozone catalyst. However, ozone tends to be thicker in higher latitudes and thinner in the equator.
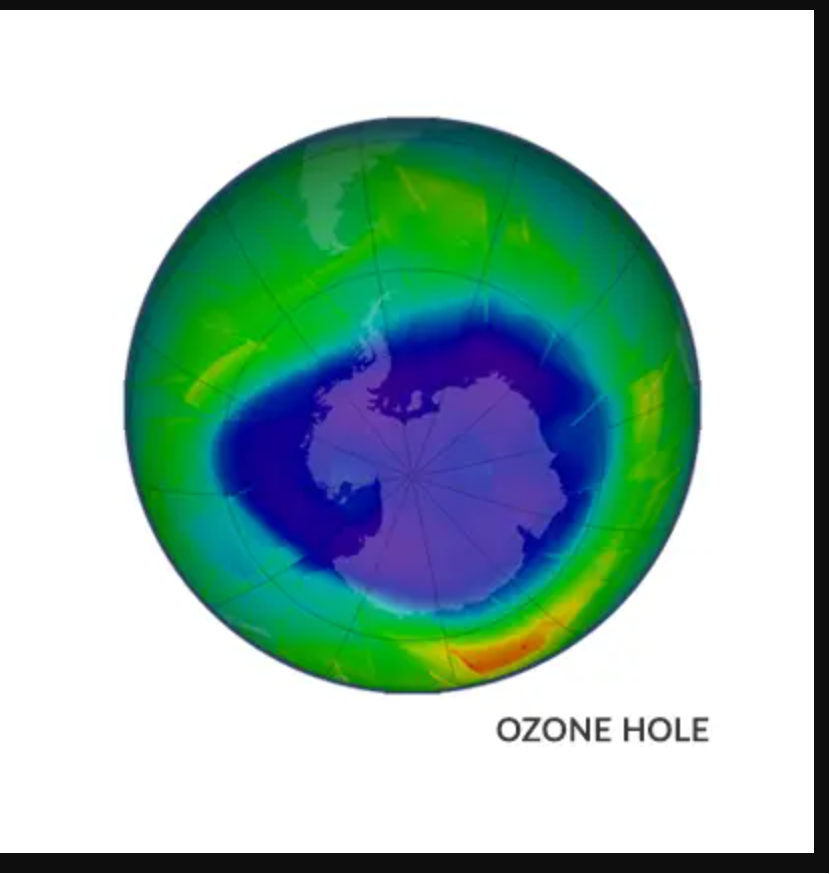
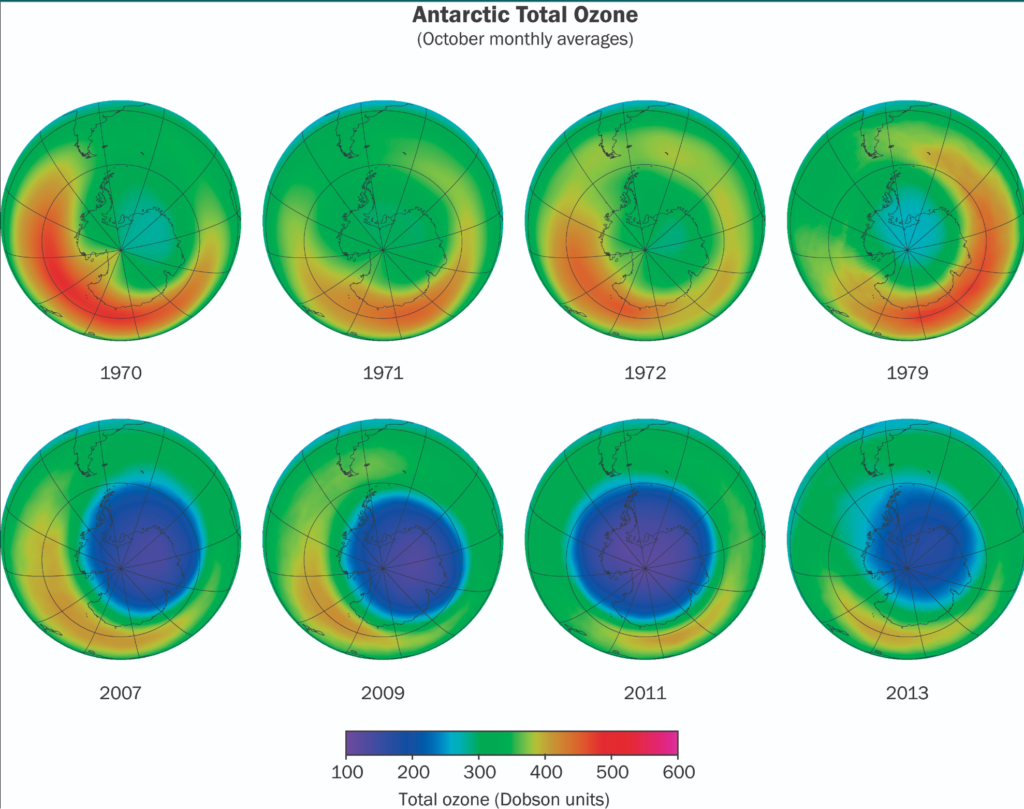
In fact, the equator produces the most ozone because of the increased UV light there. However, ozone is driven poleward by stratospheric circulation patterns. Column ozone over the Arctic and Antarctic is the exception. We refer to the significant ozone depletion that occurs in these polar regions during the winter and spring as the “ozone hole.”
Why is the ozone layer depleting?
Chlorofluorocarbons (CFCs) were utilised in air conditioners, refrigerators, and aerosol cans in the 1980s. Although many have been phased out for environmental protection reasons, certain items still use CFCs today. The primary issue of CFCs is that
ENTRY INTO ATMOSPHERE: The atmosphere is exposed to CFCs.
OZONE DEPLETION :Next, CFC is broken down into chlorine by UV light. Ozone is removed from the environment by chlorine, which acts as a catalyst to convert ozone into O2.
There is a lot of ozone depletion in Antarctica. While it is there, it is less noticeable in the Arctic. Because the geographic poles offer the best conditions, this phenomena happens there.
In winter, the Antarctic air is isolated by the polar vortex. Ice clouds can occur because of the bitterly cold temperature. Within the ice clouds, chlorine molecules link together. Finally, as the temperature rises, ozone is broken down into O2. This causes the ozone layer to break.
When did the ozone layer form in Earth’s history?
You need both oxygen and ultraviolet light to form an ozone layer.
Furthermore, the ozone layer is exclusive to Earth since no other planet in our solar system has oxygen.
Life was limited to shallow water prior to the formation of the ozone layer. This is so that dangerous radiation is shielded by water.
Life on Earth could finally diversify once ozone began shielding the planet from lethal UV radiation.
Earth’s ozone layer
An area of the stratosphere known as the Earth’s ozone layer is where ozone (O3) molecules are concentrated in relatively high concentrations.
It is essential for absorbing and preventing most of the sun’s harmful ultraviolet (UV) radiation, shielding life on Earth from the damaging consequences of prolonged UV exposure.However, experts are quite concerned about ozone depletion, which is one of the most urgent environmental challenges of our day.
Concerning the ozone layer on Earth, do you have any questions? Kindly inform us by leaving a comment below.
Also read : Gases In The Atmosphere : What Is The Reason For The Absence Of Mass-Based Gas Separation In The Atmosphere?




































