Introduction : color Atom
The concept of color is a fundamental aspect of our perception of the world. From the vibrant hues of a sunset to the rich colors of a painting, we are surrounded by a visual spectrum of shades. But what about the tiniest building blocks of matter – atoms? Do atoms possess color, or are they truly colorless? In this article, we embark on a journey into the subatomic realm to explore the intriguing question of whether an atom has a color.
The Quest for Atom’s Color
Atoms, often described as the basic units of matter, are incredibly small, with a typical size on the order of angstroms (10^(-10) meters). Due to their minuscule size, atoms are well below the resolution of the human eye, and we cannot directly see them in the traditional sense. As a result, the question of an atom’s color takes us into the domain of scientific inquiry.
The Nature of Light and Color

Color in the world around us arises from the interaction of light with objects. When white light, such as sunlight, strikes an object, it contains a spectrum of colors, each corresponding to a different wavelength of light. The color we perceive is the result of the wavelengths that are absorbed or reflected by the object.
Also read : Double Entanglement: A Glimpse Into Quantum Physics’ Intriguing Phenomenon
The Color of Atoms
Atoms interact with light primarily by absorbing or emitting specific wavelengths. Each element is characterized by a unique set of electronic energy levels, and when an atom’s electrons transition between these energy levels, it can absorb or emit light of specific colors. These colors are known as the atom’s spectral lines.
Spectral Lines: An Atom’s Signature
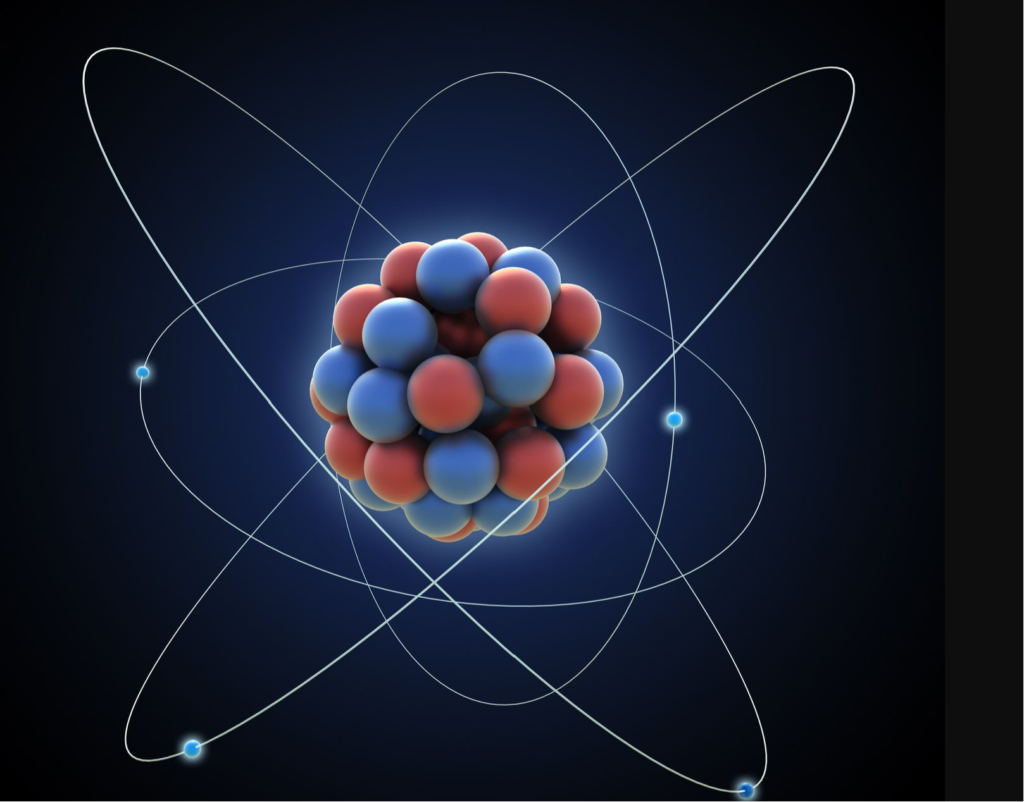
When we observe the spectral lines of elements, we see them as specific colors in the form of lines on a spectrum. For example, the element hydrogen emits light in the red and blue regions of the spectrum, giving it distinct spectral lines. These lines are the “colors” of hydrogen, in a sense.
The Limitation of Human Perception
Here’s where it gets intriguing. While we can describe the spectral lines of atoms in terms of colors, these colors are not necessarily the same as the colors we perceive in our everyday world. Atoms emit and absorb light at wavelengths that may not fall within the visible spectrum for human vision.
The Ultraviolet and Infrared Realm

Many spectral lines emitted or absorbed by atoms fall outside the range of human vision. Some are in the ultraviolet (UV) region, while others are in the infrared (IR). Humans cannot see UV or IR light without specialized instruments, so these colors are invisible to us.
The Art of Spectroscopy
The study of an atom’s spectral lines is made possible by a field known as spectroscopy. Spectroscopy involves the analysis of the light emitted or absorbed by atoms and molecules. Scientists use specialized instruments like spectrometers to record these spectral lines. These tools can detect wavelengths far beyond the limits of human vision, revealing the complete spectrum of colors an atom interacts with.
The Quantum Dance of Electrons
Understanding an atom’s colors requires delving into the quantum realm. In quantum mechanics, electrons orbit the nucleus in specific energy levels or orbitals. When an electron transitions from a higher energy level to a lower one, it emits light with a specific wavelength, giving rise to a spectral line. Conversely, when an atom absorbs light of a specific wavelength, it can promote an electron to a higher energy level.
The Unique Palette of Elements
Each element in the periodic table has its own unique set of spectral lines. These lines can be thought of as the element’s signature colors. For instance, sodium vapor emits a brilliant yellow color, which is used in streetlights, while neon produces the well-known vibrant red glow seen in neon signs.
Exploring the Invisible Spectrum
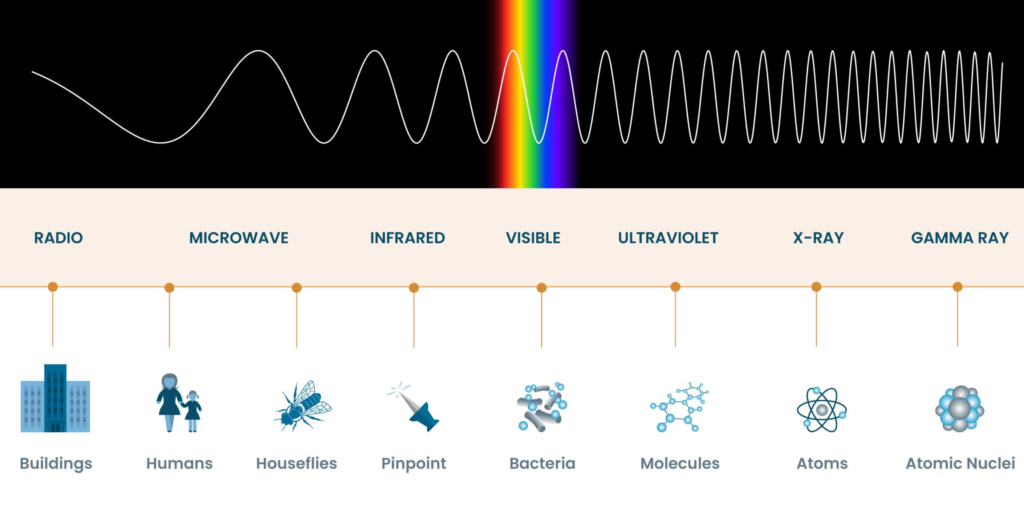
While some spectral lines correspond to colors within the visible spectrum, many fall outside the range of human perception. Ultraviolet and infrared wavelengths, which are invisible to our eyes, are a common domain for spectral lines. This fact reveals that the colors of atoms are not always confined to the familiar ROYGBIV spectrum.
The Artistic Intersection of Science and Perception
The question of whether atoms have color provides a captivating glimpse into the intersection of science and human perception. While atoms indeed possess their own unique “colors” in the form of spectral lines, these colors may exist beyond our natural vision. Our ability to “see” atoms relies on advanced scientific instruments that can translate the invisible colors of the atomic world into something comprehensible to our senses.
Conclusion: A World of Hidden Beauty
The colors of atoms, hidden within the realm of quantum physics and spectroscopy, are a testament to the captivating beauty of the natural world. Atoms may not have colors in the way we commonly perceive objects, but they possess their own remarkable palette of spectral lines. The study of these spectral lines allows us to unveil the hidden colors of the subatomic universe, revealing the intricate dance of electrons and the unique “colors” of each element. This journey into the world of atoms and their colors serves as a reminder that the universe’s beauty often lies beyond the limits of our naked eye and depends on the interplay of science and human perception.
Also read : The Unseen Shadows: Can Air Cast Invisible Silhouettes?




































