Geology is a science that delves deep into Earth’s history by Carbon Dating the rocks that form its crust. One of the key questions geologists seek to answer is the age of these rocks. To accomplish this, they employ various dating methods, one of which is carbon dating. In this article, we will explore how geologists use carbon dating to unveil the age of rocks and the valuable insights it provides into our planet’s history.
Carbon Dating: An Overview

Carbon dating, also known as radiocarbon dating, is a widely used method for determining the age of organic materials containing carbon. It relies on the radioactive decay of the carbon isotope carbon-14 (14C). While this dating technique is typically used on organic remains like fossils, wood, and bones, geologists have found innovative ways to apply it to rocks.
The Principle of Carbon Dating
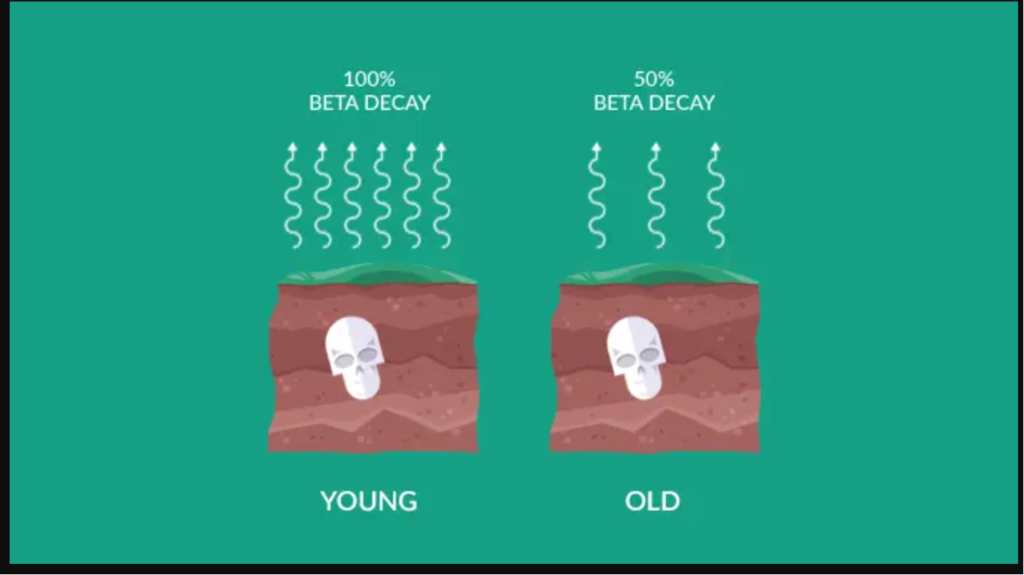
Carbon-14 is a radioactive isotope of carbon that is formed in the upper atmosphere through cosmic ray interactions with nitrogen-14 (14N). Living organisms, including plants and animals, absorb carbon-14 from the atmosphere as long as they are alive. Once an organism dies, it no longer takes in carbon-14, and the isotope begins to decay at a known rate. By measuring the remaining carbon-14 in a sample and comparing it to the initial amount, scientists can calculate the time that has elapsed since the organism’s death.
Also read : Deadly Dust: The True Cause Of The Dinosaur Extinction Following The Asteroid Impact
Applying Carbon Dating to Rocks
While carbon dating is typically used on organic materials, geologists can indirectly determine the age of rocks through a process called carbonization. Here’s how it works:

- Incorporation of Carbon: Some rocks contain small amounts of organic material, like plant debris or microscopic fossils. Over time, carbon-14 from the atmosphere can be incorporated into this organic material.
- Measurement of Carbon-14: Geologists can extract the organic material from the rock and measure the amount of carbon-14 remaining. The ratio of carbon-14 to carbon-12 in the sample provides a clue to its age.
- Age Calculation: By comparing the measured carbon-14 ratio to the initial atmospheric ratio, geologists can estimate the age of the rock containing the organic material.
Limitations and Challenges
Using carbon dating on rocks has its limitations. It is generally effective for relatively young rocks, as the carbon-14 isotope has a relatively short half-life of about 5,730 years. Therefore, it is most useful for rocks with ages up to tens of thousands of years. For older rocks, geologists turn to other dating methods, such as uranium-lead dating.
Significance for Geology
Carbon dating, when applicable, provides geologists with a valuable tool to date rocks and gain insights into Earth’s history. It helps in understanding the chronology of geological events, such as the formation of sedimentary layers, volcanic eruptions, or the age of fossils embedded in rocks. This information is crucial for reconstructing Earth’s history and the evolution of life on our planet.
Challenges in Rock Carbon Dating
While carbon dating is a powerful tool for dating rocks with organic components, it’s important to acknowledge the challenges and limitations faced by geologists when using this technique. Here are some of the key considerations:

- Sample Size: Obtaining a sufficient sample of organic material from a rock can be challenging, particularly when dealing with ancient rocks. In many cases, the amount of organic material available for testing is minimal, which can lead to less precise age estimates.
- Contamination: Contamination with modern carbon can occur during the sampling and preparation process. Even a small amount of contamination can significantly affect the accuracy of the age determination.
- Carbon Loss: Over geological time scales, organic materials in rocks can experience carbon loss through chemical reactions or leaching. This loss can make it difficult to accurately determine the original carbon-14 content.
- Calibration: Calibration is essential for converting carbon dating results into calendar years. Calibrating results involves comparing the measured carbon-14 ratios with known standards and requires a comprehensive understanding of the local environment, which can vary over time.
Alternative Dating Methods
In cases where carbon dating is not applicable due to the age of the rocks, geologists turn to alternative dating methods. Some of these methods include:

- Uranium-Lead Dating: This technique is used for dating rocks that are billions of years old. It relies on the decay of uranium isotopes into lead isotopes and is particularly useful for dating igneous rocks.
- Potassium-Argon Dating: Potassium-argon dating is suitable for dating volcanic rocks. It relies on the radioactive decay of potassium-40 into argon-40 and is used to determine the age of lava flows and volcanic ash layers.
- Luminescence Dating: Luminescence dating methods, such as optically stimulated luminescence (OSL) and thermoluminescence (TL) dating, are used to date minerals in sedimentary rocks and archaeological materials. They measure the trapped electrons’ release when minerals are exposed to heat or light.
In Conclusion
Carbon dating has expanded its application from organic materials to rocks, enabling geologists to unlock a wealth of information about Earth’s history. While it is not the primary method for dating all rocks, it offers valuable insights into more recent geological events and helps us understand the timing of important processes like fossilization, sedimentation, and volcanic eruptions. In conjunction with other dating methods, carbon dating enriches our understanding of Earth’s chronology and contributes to the continuous unraveling of the planet’s intricate geological story.
FAQ
1. What are the primary materials geologists can date using carbon dating?
Carbon dating is primarily used to date organic materials containing carbon, such as wood, bone, shell, and fossilized organic remains. In the context of rocks, geologists can date rocks that contain traces of organic materials through a process called carbonization.
2. What is the time range for which carbon dating is effective?
Carbon dating is most effective for relatively young materials, typically up to tens of thousands of years. This is because the carbon-14 isotope used in carbon dating has a relatively short half-life of about 5,730 years.
3. Can carbon dating be used to date all types of rocks?
Carbon dating is not suitable for all types of rocks. It is most effective for rocks that contain organic components, such as plant debris or microscopic fossils. For dating older rocks or those without organic material, geologists turn to alternative dating methods like uranium-lead dating or potassium-argon dating.
4. How do geologists account for potential contamination when using carbon dating?
Geologists take great care to prevent contamination during the sampling and preparation of materials for carbon dating. They use rigorous procedures, clean equipment, and may also use background measurements to identify and correct for any contamination that may have occurred.
5. What is the significance of carbon dating for understanding Earth’s history?
Carbon dating, when applicable, provides crucial information for understanding the timing of geological events and the evolution of life on Earth. It helps geologists piece together the history of our planet, from the formation of sedimentary layers to the age of fossils embedded in rocks.
6. Are there any other dating methods used by geologists besides carbon dating?
Yes, geologists use a variety of dating methods depending on the type and age of the rocks or materials they are studying. Some of these methods include uranium-lead dating, potassium-argon dating, luminescence dating, and more. Each method has its own range of effectiveness and is chosen based on the specific geological context.
Also read : Charles Darwin And Natural Selection In The Theory Of Evolution




































