Water is a unique substance that plays a central role in life on Earth. One of its intriguing properties is its ability to exist in three distinct phases: solid, liquid, and gas. The freezing point of water, where it transitions from a liquid to a solid state, is commonly understood to be 0 degrees Celsius (32 degrees Fahrenheit). But can water defy this conventional wisdom and remain in a liquid state at temperatures below freezing? In this article, we will explore the conditions under which water can stay liquid at sub-freezing temperatures and the science behind this phenomenon.
Supercooling: The Key to Liquid Water Below Freezing
Supercooling is the phenomenon that allows water to remain in a liquid state at temperatures below its freezing point. This occurs when water is cooled well below 0 degrees Celsius (32 degrees Fahrenheit) without undergoing the phase transition to ice. Several factors influence supercooling:
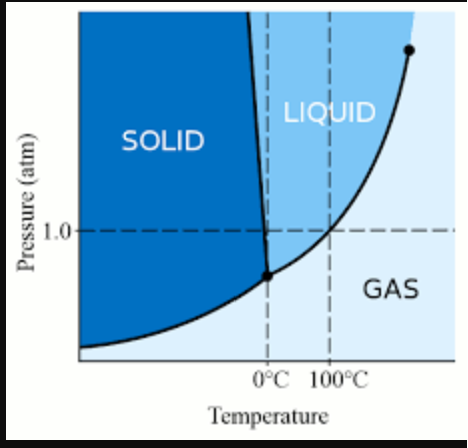
- Purity of Water: Highly pure water, with minimal impurities and dissolved gases, is more prone to supercooling. Impurities can act as nucleation sites, triggering the formation of ice crystals.
- Lack of Agitation: Still or undisturbed water is more likely to supercool because mechanical disturbances can encourage ice crystal formation.
- High Pressure: Increasing the pressure on water can lower its freezing point, allowing it to remain liquid at colder temperatures. This is the principle behind the salt-ice mixture used in ice cream makers.
- Nucleation Sites: Even in supercooled water, the process of freezing will eventually begin when an ice nucleation site is introduced, such as a speck of dust, a crystal, or even tapping the container. Once initiated, ice formation propagates rapidly.
Also read :The Sun: Our Closest Star And The Source Of Life
Natural Examples of Supercooling
While supercooling is a fascinating laboratory phenomenon, it can also be observed in nature. For example:

- Supercooled Raindrops: In some weather conditions, raindrops can exist in a supercooled state, remaining liquid as they fall through sub-freezing air. When these supercooled raindrops come into contact with a surface, like a car windshield, they freeze instantly.
- Frost Formation: Supercooling can also lead to the formation of frost. When supercooled water droplets in the atmosphere come into contact with surfaces below freezing, they freeze and create frost patterns.
Applications of Supercooling
Supercooling has practical applications in various fields, including cryogenics and food preservation. For example:

- Cryopreservation: Supercooling is used to preserve biological samples, tissues, and even embryos at extremely low temperatures without causing cellular damage.
- Food Preservation: Freezing and supercooling are techniques employed in the food industry to extend the shelf life of products like ice cream, maintaining a smooth texture and preventing ice crystal formation.
Conclusion
Water’s ability to remain in a liquid state at temperatures below freezing is a fascinating aspect of its behavior. Supercooling, influenced by factors like purity, pressure, and the absence of nucleation sites, allows for this phenomenon. While supercooled water can exist naturally in specific conditions, it is also harnessed for practical applications in various fields. Understanding the science of supercooling not only deepens our knowledge of water but also provides valuable tools for science and technology.
FAQ
1. Can I supercool water at home?
Yes, it’s possible to supercool water at home, but it can be a bit challenging. You’ll need very pure water, a clean container, and a freezer with stable temperatures. Even with these conditions, it may not always work, as supercooled water can freeze spontaneously when disturbed.
2. Why doesn’t supercooled water freeze immediately upon cooling below freezing?
Supercooling occurs when water is in a state of thermodynamic instability. While the temperature is below freezing, the transition to a solid state requires the formation of ice nuclei, which can be a rare occurrence in pure, undisturbed water.
3. Can supercooled water be dangerous?
Supercooled water can be a hazard in certain situations. For example, supercooled raindrops can freeze upon contact with roads, creating black ice, which is highly slippery and poses a danger to drivers and pedestrians.
4. Are there any practical applications for supercooled water in daily life?
Supercooling is not typically used in everyday life but has specific applications in fields like cryopreservation, where biological samples and tissues need to be stored at very low temperatures without damage.
5. What happens if you disturb supercooled water, and it freezes suddenly?
If you disturb supercooled water, causing it to freeze spontaneously, you’ll see ice crystals forming rapidly throughout the container. This can be a fascinating experiment to observe but should be done with care, as the sudden release of latent heat can lead to a burst of crystallization.
6. Can supercooled water stay in a liquid state indefinitely?
In theory, supercooled water could remain in a liquid state indefinitely if kept pure, undisturbed, and well below its freezing point. However, in practice, it is challenging to maintain these conditions, and ice formation is likely to occur over time.
7. How do antifreeze agents prevent freezing in supercooled liquids?
Antifreeze agents, such as those used in car radiator systems, lower the freezing point of a liquid. They inhibit ice crystal formation and promote supercooling, allowing the liquid to remain in a liquid state at lower temperatures than it would naturally.
8. Is it possible to drink supercooled water?
Drinking supercooled water is safe, as long as the water itself is pure and uncontaminated. However, the risk lies in disturbing the water during the process, which can cause it to freeze suddenly and may lead to unintended consequences.
Also read : Charles Darwin And Natural Selection In The Theory Of Evolution




































